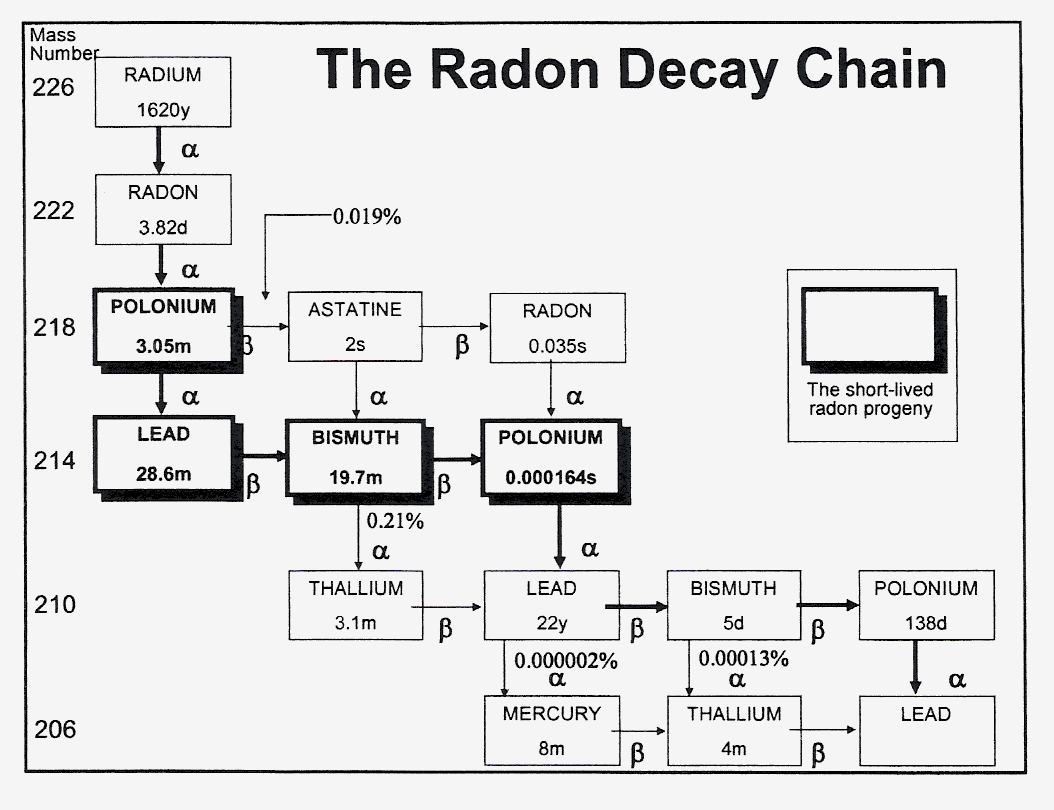
 Fig. 1. The radon decay chain (Darby, 2006)
Fig. 1. The radon decay chain (Darby, 2006)Review Article |
INTRODUCTION
For centuries or millennia spas have been important tools of medicine and preferred sites of social life.
The features, the warm water with chemical contents (inorganic minerals, salts and organic components – the messages
of the old past) build up the pleasant smell, touch, taste. These are the gifts of Nature. The analysis of the health
effects is therefore, a proper topic for environmental medicine, too. The subjects of the present review is the
significance of radon in the natural waters and consequently in the air of spas and whether the radon contributes
to the healing effects in balneotherapy. Several international conferences have dealt with these topics in the recent
decades like “Grundlagen der Radontherapie” (1979), “Radon in Bädern” (1993), “Radon Risk” (2006). Of course, the
convening of conferences was stimulated by the increasing social interest in radiation protection in the seventies and
since then (ICRP60, 1990). The radon being a radioactive noble gas and its decay products being radioactive metals
evoked wide special research and debate as carcinogenic environmental factors especially when their concentrations
enhanced technologically like in underground mines or in indoor surroundings of the population. In the context of spas
and radon, the approaches to conclude whether they are beneficial or detrimental and risky might cover the following:
– whether the presence of radon in its concentrations relevant to the spas means quantifiable risk as derived from
epidemiological data,
– whether the ionizing radiation due to the radon and its decay products in the relevant low doses means any
biological effect on the cells and organism based on radiobiological observations,
– whether the radioactivity in health spas contribute to the healing of illnesses or wellness of the patients and
visitors.
The answers concern public health, radiation hygiene and protection, radiation biology, balneology and last but not least the health tourism. Accordingly, the present review provides data – far from being complete – to guide the interested reader and stimulate further research and studies.
BASICS ON RADON
Radon is a colourless, odourless and tasteless gas produced by radioactive decay of uranium and thorium. There
are two main isotopes of radon in nature: – 222Rn (T1/2=3.82d) and its short-lived decay
products: 218Po, 214Pb, 214Bi, 214Po, 210Pb, 210Bi,
210Po (uranium series), – 220Rn (T1/2=55.6s, also called thoron) and its decay
products: 216Po, 212Pb, 212Bi, 212Po, 208Tl (thorium series).
Radon is found in most earth materials, in the atmosphere, in ground water and even in drinking water. It can enter
building materials in a number of ways. Large quantities of radon are found in many homes due to the break and stones
often have large radioactive backgrounds caused by radon. The radon content of outdoor air 1 metre above ground
typically gives 4 to 15 Bq·m–3. The average indoor air concentration of radon varies from location to
location, depending upon the uranium and thorium content and physical characteristics of the soil, moisture, winds
and building materials. In most countries the average indoor radon concentration is a few tens of Bq·m–3,
however, during the surveys hundreds and even thousands could be found. The International Commission on Radiological
Protection (ICRP) therefore recommended action levels 200–600 Bq·m–3 for homes and 500-1500 Bq·m–3
for workplaces which correspond to annual doses of 3–10 mSv in either case (ICRP65, 1993).
The radioactive decay chain is shown in Fig 1.
(Darby, 2006). From health risk aspects mostly the short-lived progenies are important, i.e. those alpha-emitting
nuclides which have short physical half-lives. The alpha particles in tissues have a path of a few tens of
micrometers (Table 1; Hofmann and Steinhäusler, 1979). Accordingly, either nuclide is
involved it is deposited in the bronchi or attached to the skin, irradiates the sensitive cell layers, i.e. bronchial
epithelium and epidermal basal cells, respectively. When entering the blood stream by either way the nuclides easily
deposit in the lipid material of cells and tissues irradiating the neighbouring cells. The cellular reactions depend
on the nature of the cells. It has to be emphasized that the fraction of radon or decay products reaching other cells
than the bronchial epithelium or skin epidermis is much less. Table 2 indicates these differences well (Pohl,
1979). Naturally, the less the local concentration is the less the absorbed dose is, and consequently the radiation
risk, if any, as well.
 Fig. 1. The radon decay chain (Darby, 2006)
Fig. 1. The radon decay chain (Darby, 2006)TABLE 1. The energies and path-lengths in tissues of particles of radon and decay products (Hofmann and Steinhδusler, 1979)
|
Radioisotope |
Energy, MeV |
Path-length, μm |
|
222 Rn |
5,490 |
41,1 |
|
218 Po |
6,002 |
47,1 |
|
214 Po |
7,687 |
70,4 |
|
210 Po |
5,305 |
48,9 |
TABLE 2. The alpha radiation dose in various organs and tissues (Pohl, 1979)
|
Organ or tissue |
Staying for 20 min in thermal spa* |
|
Bronchi |
61 |
|
Alveoli |
50 |
|
Blood |
4 |
|
Liver |
3 |
|
Kidneys |
5 |
|
Adrenal gland |
8 |
|
Muscle |
3 |
|
Bones |
1 |
|
Bone marrow |
4 |
|
Gonads |
5 |
RADON LEVELS IN SPAS
The average activity-concentrations of radon in the human environment cover rather wide ranges. Table 3 presents some examples (Köteles, 1993). The concentrations in soil, water and earth gas might occur in five orders of magnitude range. Beside the natural occurrences there are technologically enhanced natural environmental conditions, where the radon concentrations might be even higher than above, like man-made structures, houses, mines. Like in any environmental element, the radon concentrations of mineral and thermal waters, consequently of the spas differ in rather wide range. Examples are given in Table 4.
TABLE 3. Examples of 222Rn activity-concentration values in the human environment (Köteles, 1993)
|
Environmental element |
Range of activity-concentration |
|
Soil, in 1 m depth |
5 – 200 |
|
Air, above dry land |
2 – 10·10–3 |
|
Air, above oceans |
2 – 22·10–5 |
|
Earth gases |
10·10–3 – 54 |
|
Indoor* |
0,002 – 100 |
|
Uranium mines* |
10 – 103 |
|
Tunnels |
0,2 – 2 |
TABLE 4. Examples of 222Rn activity-concentrations in mineral and thermal waters
|
Type of water |
Bq·L–1 |
References |
|
Wells |
10 – 105 |
Köteles, 1993 |
|
Hungarian drinking water |
0,9 – 14,1 |
Szerbin and Köteles, 2002 |
|
Eger |
2 – 95 |
|
|
Mátraderecske |
30 – 350 |
|
|
Hévíz |
6,8 ± 2,2 |
|
|
Austria drinking and mineral water |
0,05 – 700 |
Schönhofer, 1989 |
|
Slovenia mineral water |
0,2 – 63 |
Kobal et al, 1979 |
|
Slovenia surface water |
0,09 – 5,4 |
|
|
Hungarian spas |
Szerbin et al, 1994 |
|
|
Rudas thermal mineral |
67 – 366 |
|
|
Gellért mineral |
52 – 132 |
|
|
Hévíz lake at spring |
4,63 ± 0,82 |
|
|
Hévíz lake at outlet |
3,83 ± 0,58 |
Szerbin, 1996 |
|
Lukács spa |
22 – 61 |
|
|
Romania |
Mócsy, 2005 |
|
|
Tusnad spa |
6,9 ± 1,0 |
|
|
Felix spa |
12,9 ± 1,9 |
|
|
Marghita spa |
5,7 ± 1,0 |
|
|
Kovasna spa |
0,3 – 613 |
|
|
Hargita spa |
10 – 13 |
The air in various rooms of spas has also a wide range of activity-concentration
of radon which is inhaled by visitors and workers. For example, the values in the mentioned spas are as follows:
DOSE ASSESSMENTS FROM RADON EXPOSURE
From the radon concentration in air the effective dose values can be assessed (Dombóváry et al., 2006) by taking into consideration the activity-concentration (CRn in Bq·m–3), the dose conversion factor (K) from the inhaled amount to sievert (7.9 · 10–9 Sv · (Bq·m–3)–1 · h–1), the equilibrium factor (f) of radon and decay products (usually 0.4) and the time length of staying in the respective room (T), i.e.
The radiation dose from drinking radon-containing water can be assessed similarly as follows.
where
K is the conversion factor for ingested water (3.5 · 10–9 Sv · (Bq·L–1)–1),
G is the amount of water taken up (L),
c is the activity-concentration of water (Bq·L–1).
The effective dose values can also be assessed for persons having a bath course. A few examples of results of
such calculations are as follows: The radiation doses to cells and tissues from environmental radon
activity-concentrations can also be assessed by the exposure, exposure rate, time length of exposure usually by
models. An example is given (Maushart, 2005) suggesting that when the water source contains 3256 Bq·L–1,
then in the water of the bath-tub there is only 1800–2000 Bq·L–1. Accordingly, a healing course
(10 occasions, 20 minutes per each) results in 2 mGy to the surface of the skin, 30 µGy to the whole skin and a few
µGy dose for the other tissues like blood. The latter contained under such conditions 4 Bq·L–1, the exhaled
air contained 2.5 Bq·L–1. Beside the exposures from the water and air in the spa itself, the patients
staying in the various rooms of the health centres might get even higher exposure than in the basins (Pohl, 1979).
The committed effective dose assessment of visitors in Hévíz Lake spa in case
of every day bathing is between 0.7–0.9 mSv per year (Committed effective dose is the effective dose to tissue over
an integration time elapsed after an intake of radioactive substances). The patients’ effective dose in the hospital
is not more than 102 µSv and in the health hotel 34 µSv (Dombóváry et al., 2006). These values are due to the
radon activity-concentrations in air, i.e. between 340–625 Bq·m–3 in various rooms.
It is known since the beginning of such calculations that mostly the air radon
concentration contributes to the radiation dose by inhalation and that the professional staff is exposed for longer
time than the visitors and effective doses what they get are a few – 0.2 to 15 – mSv per year (Pohl, 1979; Mócsy, 2005).
In a “mofetta” in Kovasna, the radiation burden for staying for 30 minutes with
11.1 Bq·L–1 radon air concentration is appr. 200 µSv (Szabó, 1998). In Rudas spa the visitors’ committed
effective doses are between 0.8–1.7 mSv per year (Szerbin, 1996).
It is obvious that the excess radiation burden from spas is less than the annual
natural background in which all persons live during the whole life. Table 5 summarizes the
average radiation doses from natural sources.
TABLE 5. Average worldwide exposure to natural radiation
|
Source |
Annual effective dose (mSv) |
|
|
Average |
Typical range |
|
|
Cosmic rays |
0,39 |
0,3 – 1,0 |
|
External terrestrial |
0,48 |
0,3 – 0,6 |
|
Inhalation (mainly radon) |
1,15 |
0,2 – 10 |
|
Ingestion |
0,29 |
0,2 – 0,8 |
|
Total |
2,4 |
1 – 10 |
RISK OF RADON INHALATION
The adverse health effects of exposure to radon are caused primarily by damage due to alpha-particles. The
possible effects will depend on the exposure level. The main danger from high radon exposure is an increased risk of
lung cancer. Radon as a noble gas is rapidly exhaled after being breathed in; however radon progenies combine with
other molecules in the air and with particles of dust, aerosols or smoke, and readily deposit in the airways of the
lung. While lodged there, the progenies emit ionizing radiation in the form of alpha particles, which can damage the
cells lining the airways, i.e. bronchial epithelial cells where they could initiate cancer. In human population the
epidemiological studies on thousands of uranium miners in different countries support this fact (UNSCEAR, 2000).
But exposure to radon in houses can also lead to lung cancer (WHO, 2002). Lung cancer risk, however, is several
times higher when radon exposure is combined with smoking. It is believed that the relationship between radon and
risk of lung cancer is linear. In other words, doubling the exposure doubles the risk and halving the exposure halves
the risk. Lung cancer risk from residential radon exposure is substantially lower since the exposure in homes is much
lower than in mines, although the risk increases with radon concentration level and duration of exposure. For life-time
exposure to radon of 20 Bq·m–3 level at home the risk of lung cancer is estimated to be 0.3% (or 3 death in
1000 people). For comparison, risk of accidental death at home is 0.7% (or 7 in 1000) (WHO, 2002). A recent large
meta-analytical study of epidemiological investigations on the risk of indoor radon concluded that the risk estimate
for lung cancer increased by 16 per cent per 100 Bq·m–3 when such concentration is involved in dwellings
almost all the day and many years (Darby, 2006). In the risk assessments beside the concentration, the time-length also
has to be considered. In spas the people stay for much less time than in their homes.
It has been suggested that other effects of radon exposure include increased
risk of non-malignant respiratory diseases but this is much less clearly established than the lung cancer risk. It is
still not clear whether children are more sensitive to radon exposure. Studies on childhood leukaemia (the most common
form of cancer in childhood) have not found clear evidence of risk associated with radon concentrations in homes.
THE LOW DOSE EFFECTS
The biological effects of ionizing radiation for radiation protection considerations are grouped into two categories according to their dose-effect relationships: the deterministic and the stochastic ones (Fig 2). The former ones are caused by high doses, the latter ones by low doses. The stochastic effects might occur following several tens of mSv. The probability of consequences increases with the dose and the relationship between dose and effect is assumed to be linear. Accordingly, not having a “threshold” dose a certain risk – albeit very small – can be attributed to any low dose.
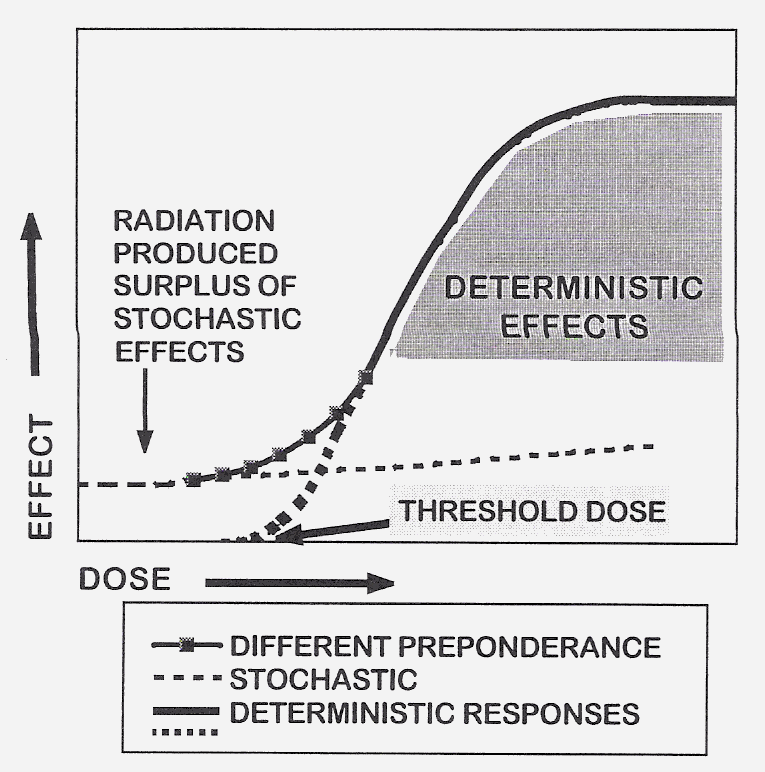 Fig. 2. Schematic dose-response curves for the stochastic
Fig. 2. Schematic dose-response curves for the stochastic The model for assessing the detrimental health effects used for the stochastic
effects is the linear-non-threshold “L-NT” one. In the low dose dilemma the problem raised is whether the use of the
L-NT model is justified to attach any health risks to low doses as the risk of low-level exposure to ionizing radiation
is uncertain and a single extrapolation from high-dose effects may not be wholly justified in all instances (Köteles,
1998; ICRP99, 2006). Based on epidemiological data of radiation-induced cancer occurrences, various authors agree that
low dose is below 200 mGy as under this level the statistical evaluation of data becomes more and more uncertain
(UNSCEAR 1994; Heidenreich et al., 1997; Tubiana, 2003). Accordingly, based on the frequency of cancer cases the
extrapolation of risks from high doses to low ones is not justified.
Among the low dose radiation-induced cellular alterations recently special
interest has been focussed towards the hormesis and adaptive responses. These phenomena, i.e. inducing stimulatory or
beneficial effects have more and more become the targets of research.
RADIATION HORMESIS
The term refers to a process whereby low doses of ionising radiation may result in beneficial or stimulatory effects. The underlying property is a physiological effect that cannot be anticipated by linear downward extrapolation from the toxic levels of exposure. There is a large body of literature, supported by statistically significant epidemiological studies, that speaks in favour of radiation hormesis, which have demonstrated that exposure to low level irradiation has apparently resulted in positive health effects (Kant et al., 2003; Mortazavi et al., 2006; Averbeck et al., 2006). The hormetic argument that whole body exposure to low level irradiation may actually decrease cancer risk is based primarily on the analysis of occupational and environmental data of various related studies (Kant and Chakarvarti, 2006). Reports exist on various epidemiological studies demonstrating a negative correlation of lung cancer risk with radon in dwellings, which shows that exposure to low level ionising alpha radiation has apparently resulted in positive health effects (Averbeck et al., 2006). Studies show that there is a protective effect of radon in the range of appr. 20–200 Bq·m–3 (LáZázr et al., 2003; Kant and Chakarvarti, 2006). All these advocate the non-linearity of the dose-response curve and speak in favour of the protective effect of low-dose exposures and even indicate also that radiation hormesis exists. As possible explanation to this interesting phenomenon, it has to be considered that low doses of ionising radiation may induce or activate DNA repair functions, immune responses, anti-tumour defence systems, and detoxification mechanisms, the so-called adaptive responses with the result that there are fewer cancer deaths in individuals exposed to low level irradiation (Cameron, 1992; Pollycove and Feinendegen, 1999). Furthermore, the argument that all radiation is harmful even down to ambient levels is based upon extrapolation of data with high doses and with virtually no acceptable data with low doses. These scaled epidemiological extrapolations are invalid and the majority of the available data on low level irradiation suggest benefits (Luckey, 1999).
ADAPTIVE RESPONSE
The term ‘adaptive response’ is used to refer to the possibility that a prior exposure to a small dose of radiation, which is variously referred to as the conditioning, adapting or priming dose, may mitigate the severity of the effect due to a subsequent high dose or challenging dose of ionising radiation. Studies have established the existence of an adaptive response to radiation in human lymphocytes, pre-irradiating cells with about 0.01 Gy of low LET radiation which protects them from a subsequent dose of about 1 Gy, as measured by a lower yield of chromosome aberrations, genetic mutations, cell transformation and resistance to cell death (UNSCEAR 1994; Köteles, 2004). The protection is probably mediated by newly synthesised enzymes involved in DNA repair or in antioxidant processes. Beside the experimental observations the existence of adaptive response was demonstrated in lymphocytes of human population (Bognár et al., 2007).
BIOCHEMICAL-CLINICAL OBSERVATIONS ON THE BENEFICIAL EFFECTS OF RADON SPAS
Radon effects on cells
There is more and more information cumulating on the effects of radon at cell biological level. The gene
activity of p53 cancer suppressor gene increases to double value at a triple concentration of natural radon in
a Japanese region, as well as the superoxid dismutase (SOD) enzyme activity increases by 15 per cent (Ma et al.,
1996; Yamaoka et al., 2005). Similarly the increase of this antioxidant enzyme activity was observed during
balneotherapy and parallelly an other positive effect, i.e. decrease of serum lipids (Mitsunobu et al., 2003).
Rather early, the stimulation of DNA repair was observed upon radon exposure
(Altmann and Tuschl, 1978). Similar DNA repair was indicated in lymphocytes of people living in increased radon
concentration and also the adaptive response reaction was provoked under 10 mSv “priming” dose (Masoomi et al., 2006).
Clinical aspects: radon as medicine
The spas evidently containing radon have been used with success for hundreds of years for special illnesses mainly in the pain therapy of chronic rheumatic illnesses (Table 6; Deetjen et al., 2004; Meara, 2006). Radon spas are wide-spread in Europe (Germany, Austria, Czech Republic, Hungary, Romania, Slovenia, etc). Clinical experience has shown that the long-lasting pain of the patients was considerably reduced with less analgetic pharmaceuticals. The reduction of anti-rheumatic drugs is important in the prevention of side effects like gastric bleeding, duodenal ulcer up to the perforation (Rühle, 2005). The cause behind seems to be a regulatory change of cellular reaction in the inflammatory tissues. This is evoked by alpha-radiation like following UV-B exposure or x-irradiation. The mechanism could be that the alpha irradiated dying cells release cytokines like TGFß which inhibits the inflammation. In the radon-treated patients with Morbus Bechterew the TGFß could be detected. Another speculation on the pain relief is, though not well understood, that the action potential of the pain neural network is increased in response to the internal radon radiation field (Lykken et al., 2005). Thus the chronic pain signals are ignored since they can not sum sufficiently to trigger the new activation threshold for the pain response of neural network system. The long-lasting effects are due to the fact that the radon within the organism is stored in the lipids of the brain, blood forming organs, lymphoid tissue. The lipid storage of radon allows it to deliver the short-lived radon decay products to the neural network system continuously over periods of several weeks to months. Further important observations concern the stimulation of endocrine system especially the secretion of adrenal and thyroid glands (Phaller et al., 1979; Nagy and Berhes, 2001; Nagy et al., 2006).
TABLE 6. Indications for radon therapy (Meara, 2006)
|
Chronic pain |
|
|
Diabetes Type I & II |
Migraine Headaches |
|
Arthritis |
Eczema |
|
Mobility |
Asthma |
|
Emphysema |
Multiple Sclerosis |
|
Behcet’s |
Fibromyalgia |
|
Post Polio Syndrome |
Bronchitis |
|
Gout |
Prostate (BPH) |
|
Bursitis |
Hayfever |
|
Psoriasis |
Cancer (Breast) |
|
High Blood Pressure |
Scleroderma |
|
Carpal Tunnel |
Inflammation |
|
Sinus |
Chronic Pain |
|
Lupus (SLE) |
Ulcerative Colitis |
|
Ankylosing Spondylitis |
|
CONCLUSIONS
– Although in many sources of thermal and mineral waters the radon activity-concentrations are rather high,
by the time they reach the consumer either as drinking water, or in spas the concentrations will be considerably lower.
– Radiation protection calculations suggest that due to the exposure levels the effective doses are in the range of
the natural background.
– Epidemiological and cell biological studies indicate that the low level of irradiation causes less detriment than
it would be expected from extrapolation from high doses.
– Low level irradiations might be beneficial through stimulating cellular and tissue reactions. This might be one of
the effects of radon spas, too.
– The presence of radon in spas, accordingly, can not be considered as risky to health, just the opposite, more and
more information cumulate on its positive health effects completing the other beneficial factors present in health spas.
ACKNOWLEDGEMENT
The author highly acknowledges the interest and advice of Professor Attila Vértes, member of Hungarian Academy of Sciences.
REFERENCES
Altmann, H. and Tuschl, H. (1978). “DNA-repair investigations in lymphocytes of persons living in elevated natural background radiation areas (Badgastein).” in: Late biological effects of ionizing radiation, IAEA, Vienna.
Averbeck, D., Testard, I., and Boucher, D. (2006). “Changing views on ionizing radiation-induced cellular effects.” Int. J. Low Radiat. 3:117–134.
Bognár, G., Szalma, K., Ótós, M., and Köteles, G. J. (2007). “Adaptive response of gamma-irradiated lymphocytes in human population.” Centr. Europ. J. Occup. Environ. Med. 13:25–32.
Cameron, J. R. (1992). “Hormesis and high fliers: Radiation risks revised.” Physics Today, 45:13.
Cameron, J. R. (2006). “Cancer incidence in areas with elevated levels of natural radiation.” Int. J. Low Radiat. 2:20–27.
Darby, S. C. (2006). “Radon in homes and lung cancer risk.” In: Radon risk. www.ibcglobal.com
Deetjen, P., Falkenbach, A., Harder, D., Jöckel, H., Kaul, A., and Philipsborn, von H. (2004). “Radon als Heilmittel – Therapeutische Wirksamkeit, biologischer Wirkungsmechanismus und vergleichende Risikobewertung.” Verlag Dr. Kovac, Hamburg, 111 Seiten
Dombóváry, P., Somlai, J., Kovács, T., Torma, Á., Fridrich, T., and Kávási, N. (2006). “Assessment of radiation burden et Hévíz Lake spa.” (In Hungarian) Proc., III. Magyar Radon Fórum, pp. 33–39. Veszprém.
“Grundlagen der Radontherapie.” in Bad Münster am Stein, 1979 in Zschrift. f. Angew. Bäder- und Klimaheilk. 26:331.
Heidenreich, W. F., Paretzke, H. G., and Jacob, P. (1997). “No evidence for increased tumor rates below 200 mSv in the atomic bomb survivors.” Radiat. Environ. Biophys. 36:211–212.
Hofmann, W. and Steinhäusler, F. (1979). “Die Mikrodosimetrie der alpha-strahlung des Radon und seiner Zerfallsprodukte zur Erklärung biologischer Strahlenreaktionen auf zellularer Ebene.” Zeitschr. f. Angew. Bäder- und Klimaheilk. 26:399–429.
ICRP60 (1990). “Recommendations of the International Commission on Radiological Protection.” Annals of the ICRP, 21.
ICRP65 (1993). “Protection against Radon-222 at home and at work.” Annals of the ICRP, 23.
ICRP99 (2006). “Low-dose extrapolation of radiation-related cancer risk.” Annals of the ICRP, 35.
Kant, K., Chauhan, R. P., Sharma, G. S., and Chakarvarti, S. K. (2003). “Hormesis in humans expsed to low-level ionizing radiation.” Int. J. Low Radiat. 1:76–87.
Kant, K. and Chakarvarti, S. K. (2006). “Radiation hormesis, the validity of the linear no-threshold hypothesis.” Int. J. Low Radiat. 3:66–73.
Kasztovszky, Z., Kuczi, R., and Szerbin, P. (1996). “On the natural radioactivity of waters in Hungary.” Centr. Europ. J. Occup. Environ. Med. 2:335–347.
Kobal, I., Kirstan, J., Ancik, M., Jerancic, S., and Skofljanec, M. (1979). “Radioactivity in thermal and mineral springs in Slovenia.” Health Phys. 37:239–242.
Köteles, G. J. (1993). “Radon in our Environment.” (In Hungarian) OMIKK Publ. Budapest, 28 pages
Köteles, G. J. (1998). “The low dose dilemma.” Centr. Europ. J. Occup. Environ. Med. 4:103–113.
Köteles, G. J. (2004). “Through the hills and valleys of radiation biology in Hungary.” Centr. Europ. J. Occup. Environ. Med. 10:202–226.
Köteles, G. J. (2006). “Biological responses in low-dose range.” Int. J. Low Radiat. 3:97–110.
Lázár, I., Tóth, E., Marx, G., Cziegler, G., and Köteles, G. J. (2003). “Effects of residential radon on cancer incidence.” J. Radioanalyt. Nucl. Chem. 258:519–524.
Lőbb, H., Resch, A., Horváth Á., and Szabó, Cs. (2006). “Measurement of radon content in thermal water of Rudas-spa.” (In Hungarian) In: III. Magyar Radon Fórum, Pannon Egyetemi Kiadó, pp. 146–152.
Luckey, T. D. (1999). “Nurture with ionizing radiation: A provocative hypothesis.” Nutrition and Cancer, 34:1–11.
Lykken, G. I., Momcilovic, B., and Ward, T. W. (2005). “Possible underlying physics behind pain relief received in radon health spas.” Health Phys. 89/1, S16–S17.
Ma, J., Yonehara, H., Ikebuchi, M., and Aoyama, T. (1996). “Effect of radon exposure on superoxide dismutase (SOD) activity in rats.” J. Radiat. Res. 37:12–19.
Masoomi, J. R., Mohammadi, S., Amini, M., and Ghiassi-Nejad, M. (2006). “High background radiation areas of Ramsar in Iran evaluation of DNA damage by alkaline single cell gel electrophoresis.” J. Environ. Radioactivity, 86:176–178.
Maushart, R. (2005). “Biologische Wirkungen niedriger Streahlendosen.” Strahlenschutzpraxis, 11:58–60.
Meara, J. (2006). “The radon challenge – an introduction to radon, radon risks and public perception of the radon hazard.” In: Radon risk. www.ibcglobal.com
Mitsunobu, F., Yamaoka, K., Hanamoto, K., Kojima, S., Hosaki, Y., Ashida, K., Sugita, K., and Tanizaki, Y. (2003). “Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma.” J. Radiat. Res. 44:95–99.
Mócsy, I. (2005). “Radon activity concentration in rooms for mineral and thermal water treatments and in air of mofettas.” (In Hungarian) In: Radon a Kárpát-Medencében, ed.: I. Mócsy, T. Néda, Sapientia University, Kolozsvár, pp. 161–162.
Mortazavi, S. M. J., Ghiassi-Nejad, M., Karam, P. A., Skushima, T., Niromand-Rad, A., and Cameron, J. R. (2006). “Cancer incidence in areas with elevated levels of natural radiation.” Int. J. Low Radiat. 2:20–27.
Nagy, K. and Berhes, I. (2001). “Endocrine changes during radon balneotherapy.” (In Hungarian) Balneológia Gyógyfürdőügy Gyógyidegenforgalom, 22:17–25.
Nagy, K., Berhes, I., Kovács, T., Somlai, J., Kávási, N., and Laboncz, Sz. (2006). “Effect of cave-therapy on homone metabolism.” (In Hungarian) In: III. Magyar Radon Fórum, Pannon Egyetemi Kiadó, pp. 41–50.
Pfaller, W., Hofmann, W., Steinhäusler, F., and Deetjen, P. (1979). “Subzelluläre Veränderungen der Nebennierenrinde nach Inhalation von 222Radon.” Z. angew. Bäder- und Klimaheilk. 26:154–160. and 26:384–390.
Pohl, E. (1979). “Physikalische Grundlagen der Radontherapie: Organ- und Gewebedosen und ihrer Bedeutung für Patient und Personal.” Zeitschr. f. angew. Bäder- und Klimaheilk. 26:370–379.
Pollycove, M. and Feinendegen, L. E. (1999). “Molecular biology, epidemiology, and the demise of the linear no-threshold hypothesis.” C. R. Acad. Sci. III. 322:197–204.
“Radon in Bädern – Messungen und Wirkungen in Theuern.” 1993, 41. Radiometrische Seminar in Theuern, 2005, Strahlenschutzpraxis, 11:58–60.
“Radon Risk.” (2006). KE 1128 Safety and Nuclear Division, IBC Global Conferences, London, 2006, chairman: G. Kendall, www.ibcglobal.com
Rühle, H. (2005). “Radon als Heilmittel.” Strahlenschutzpraxis, 11:76–77.
Schönhofer, S. (1989). “Determination of radon-222 and radium-226 in mineral water and drinking water – a survey in Austria.” Analyst, 114:1345–1347.
Somlai, J., Horváth, G., Kanyár, B., Kovács, T., Bodrogi, E., and Kávási, N. (2002). “Concentration of 226Ra in Hungarian bottled mineral water.” J. Environ. Radioactivity, 62:235–240.
Szabó, E. (1998). “Healing factors of Kovasna natural radioactive spas.” (In Hungarian) In: Kovászna, a természet ajándéka, szerk.: Szabó Endre, pp. 68–74. Marosvásárhely.
Szerbin, P., Köteles, G. J., and Stur, D. (1994). “Radon concentration in Rudas thermal bath.” Radiat. Prot. Dosim. 56:319–322.
Szerbin, P. (1996). “Natural radioactivity of certain spas and caves in Hungary.” Environment Intern. 22:389–398.
Szerbin, P. and Köteles, G. J. (2002). “222Rn, 226Rn and U in drinking water in Hungary.” In: Technologically Enhanced Natural Radiation (TENRE II) Proc. of IAEA Symp. IAEA-TECDOC-1271. International Atomic Energy Agency, Vienna, pp. 158–165.
Tubiana, M. (2003). “The carcinogenic effect of low doses: the validity of the linear no-threshold relationship.” Int. J. Low Radiat. 1:1–33.
UNSCEAR (1994). “Epidemiological studies of radiation carcinogenesis, United Nations Scientific Committee on the effects of atomic radiation.” UNSCEAR, pp. 11–184. New York.
UNSCEAR (1994). “Adaptive responses to radiation in cells and organisms.” United Nations Scientific Committee on the effects of atomic radiation, pp. 185–272, New York.
UNSCEAR (2000). “Epidemiological evaluation of radiation-induced cancer.” Sources and effects of ionizing radiation, United Nations Scientific Committee on the Effects of Atomic Radiation, pp. 297–450.
Yamaoka, K., Mitsunobu, F., Kojima, S., Shibakura, M., Kataoka, T., Hanamoto, K., and Tanizaki, Y. (2005). “The elevation of p53 protein level and SOD activity in the resident blood of the Misasa radon hot spring district.” J. Radiat. Res. 46:21–24.
WHO (2002). “Radon and health.” www. who.int/ionizing_radiation/pub_meet/fact_press/en/print.html